
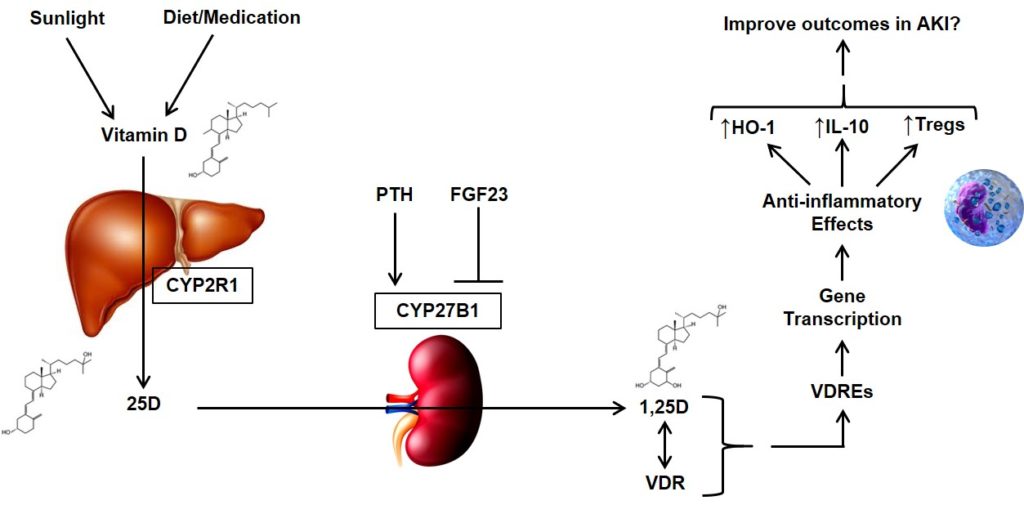
Vitamin D is derived from dietary sources and sunlight-induced cutaneous synthesis, and is converted to 25-hydroxyvitamin D (25D) in the liver. Circulating 25D is converted to its biologically active form, 1,25-dihydroxyvitamin D (1,25D), in the kidneys, immune cells, and other tissues by the cytochrome P450 enzyme, 1-α hydroxylase (CYP27B1). The conversion of 25D to 1,25D by CYP27B1 is tightly regulated by two opposing hormones: parathyroid hormone (PTH), which stimulates CYP27B1, and fibroblast growth factor 23 (FGF23), which inhibits CYP27B1. 1,25D is essential for maintaining normal calcium and phosphate homeostasis, but also has a variety of “non-classical” effects. These effects are mediated through 1,25D binding to the intracellular vitamin D receptor (VDR), which is expressed nearly ubiquitously. The 1,25D-VDR complex translocates into the nucleus, where it binds to DNA vitamin D response elements (VDREs), ultimately influencing the expression of over 200 target genes. These genes affect a variety of critical inflammatory/immunomodulatory pathways relevant to acute kidney injury (AKI).

BIOMARKER STUDIES
We established that patients with acute kidney injury (AKI) have elevated blood levels of fibroblast growth factor 23 (FGF23), an osteocyte-derived phosphaturic hormone that inhibits vitamin D synthesis. Further, we found that higher circulating levels of FGF23 and decreased levels of vitamin D metabolites are early indicators of AKI and death in a variety of clinical settings, including in hospitalized patients, in critically ill patients, and in patients undergoing cardiac surgery.
RANDOMIZED CONTROLLED TRIALS
In a randomized, double-blind, placebo-controlled trial conducted in critically ill patients with severe sepsis, we found that treatment with the activated form of vitamin D (1,25-dihydroxyvitamin D) increases leukocyte mRNA expression of hCAP18, an important antimicrobial peptide, and IL-10, a key anti-inflammatory cytokine. We are currently conducting a follow-up study, ACTIVATE-AKI: Activated Vitamin D for the Prevention and Treatment of Acute Kidney Injury. ACTIVATE-AKI is a phase II, randomized, double-blinded, placebo-controlled trial testing whether vitamin D metabolites are effective in preventing or attenuating AKI in critically ill patients.
SELECTED PAPERS FROM OUR RESEARCH
Leaf DE, Siew ED, Eisenga MF, Singh K, Mc Causland FR, Srivastava A, Ikizler TA, Ware LB, Ginde AA, Kellum JA, Palevsky PM, Wolf M, Waikar SS. Fibroblast Growth Factor 23 Associates with Death in Critically Ill Patients. Clin J Am Soc Nephrol 2018;13(4):531-41. PubMed
Leaf DE, Jacob KA, Srivastava A, Chen ME, Christov M, Jüppner H, Sabbisetti VS, Martin A, Wolf M, Waikar S. FGF23 levels are associated with acute kidney injury and death in critical illness. J Am Soc Nephrol 2017;28(6):1877-85. PubMed
Leaf DE, Christov M, Jüppner H, Siew E, Ikizler TA, Bian A, Chen G, Sabbisetti VS, Bonventre JV, Cai X, Wolf M, Waikar SS. Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney Int 2016;89(4):939-48. PubMed
Leaf DE, Croy HE, Abrahams SJ, Raed A, Waikar SS. Cathelicidin antimicrobial protein, vitamin D, and risk of death in critically ill patients. Crit Care 2015;19:80. PubMed
Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized Controlled Trial of Calcitriol in Severe Sepsis. Am J Respir Crit Care Med 2014;190(5): 533-41. PubMed
Christov M, Waikar SS, Pereira RC, Havasi A, Leaf DE, Goltzman D, Pajevic PD, Wolf M, Jüppner H. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int 2013;84(4): 776-85. PubMed
Leaf DE, Waikar SS, Wolf M, Cremers S, Bhan I, Stern L. Dysregulated Mineral Metabolism in Patients with Acute Kidney Injury and Risk of Adverse Outcomes. Clin Endocrinol 2013;79(4): 491-8. PubMed
Leaf DE, Wolf M, Waikar SS, Chase H, Christov M, Cremers S, Stern L. FGF-23 Levels in Patients with AKI and Risk of Adverse Outcomes. Clin J Am Soc Nephrol 2012;7(8):1217-23. PubMed
